Rapid antigen tests and other diagnostic techniques
Even with recent positive news on COVID 19 rapid antigen tests, it will be some time before vaccines are available to the general public. Thus, rapidly, massively, and intelligently testing, tracking, tracing, and isolating (TTTI) will continue to be critical for preventing further infection rebounds after lockdowns.
Rapid infection suppression entails testing suspected patients and all of their contacts to determine who is infected and isolating those who are; successfully following them to ensure they do not transmit the illness further, and thoroughly tracing everyone they have been in touch with.
The OECD policy brief Testing for COVID-19: A Means to Lift Confinement Restrictions, issued in May 2020, provides an overview of existing testing methods. Since then, advancements in the creation of novel testing methods and the repurposing of current technology for COVID 19 have been made, including the use of rapid antigen tests and other molecular diagnostic tools (mainly CRISPR1-based rapid antigen tests and RT-LAMP). learn more about rapid antigen test at https://clinicalsupplies.com.au/collections/rapid-antigen-tests
In contrast to a real-time polymerase chain reaction (RT-PCR), which has been the most extensively used method to date, new fast antigen testing may be simply implemented at the point of care and deliver near-instant results. While these characteristics are advantageous for optimizing TTTI techniques, rapid antigen tests, as detailed below, are less sensitive than RT-PCR, which limits their applicability in some cases. Point-of-care RT-LAMP and CRISPR-based diagnostics are gaining traction and may eventually become important additions to the “testing toolbox.”

This note updates the previous OECD brief to include current advancements in testing methods and explores the implications for more effective containment and mitigation tactics until rapid antigen tests become widely accessible. The next part describes the major technologies that are already available or anticipated in the short/medium term and summarizes them. The next section examines testing methodologies and the proper use of different technologies to facilitate them.
There are several testing technologies, each with its own purpose, features, strengths, and weaknesses.
The sole well-established method is reverse transcription-polymerase chain reaction (RT-PCR).
The reverse transcription-polymerase chain reaction (RT-PCR) is a diagnostic method for detecting viral genetic material (viral RNA) in a biological sample after it has been amplified to allow for detection. It is now the gold standard for detecting the virus’s presence in the respiratory tract, i.e. for diagnosing active infections. This approach has a high degree of sensitivity and specificity, which means it is quite trustworthy.
However, favorable outcomes might be difficult to interpret in some circumstances. Additionally, some of this method’s more basic restrictions hamper its usage on a large scale. To begin, some critical testing supplies (e.g., reagents, nose swabs, and transport medium) are in short supply.
Additionally, even if this technique is capable of returning results within hours, the logistics of sample collection, transport to a central laboratory, analysis of the sample, and return of results in a long time delay between when a sample is taken and when the results are available and communicated.
This may be a bottleneck in TTTI methods, which rely on rapidly detecting and isolating sick individuals. Finally, in certain countries, the relatively expensive cost of RT-PCR is a limitation (Carter et al., 2020[1]). To keep things simple, procedures that have many of the same properties as RT-PCR are not covered individually, including transcription-mediated amplification (TMA) and conventional RT-LAMP. These three approaches are discussed in detail in the references to RT-PCR below.
Point-of-care rapid antigen tests RT-LAMP analyses
RT-LAMP is a technology identical to traditional RT-PCR, with the difference that the nucleic acid amplification happens at a constant temperature,3 and so costly thermal cyclers used in RT-PCR are not necessary.
Until recently, RT-LAMP rapid antigen tests were done mostly in full-fledged labs and offered a comparable option to RT-PCR, but various point-of-care and near-point-of-care test kits employing this approach have been commercialized and certified for usage, including several in the EU and the US.
4 These assays demonstrate a high degree of sensitivity and specificity when used in conjunction with RT-PCR (Thompson and Lei, 2020[2]; Dao Thi et al., 2020[3]). It remains to be seen how soon the usage of RT-LAMP at the point of care can be ramped up. However, depending on the cost, the technique may prove to be a more realistic choice than antigen testing for the application in contexts such as pre-travel testing.

Rapid antigen tests based on RISPR
CRISPR-based rapid antigen tests operate by recognizing a sequence of COVID 19 viral RNA and severing any surrounding single-stranded RNA. These incisions cause the release of a fluorescent particle that was injected separately into the test fluid. When the sample is subsequently illuminated by a burst of laser light, the fluorescent particles released illuminate, indicating the presence of viral genetic material. The current prototypes using this technology offer findings within 30 minutes, are equivalent to RT-PCR in terms of performance, and may also be conducted at the point of care.
Additionally, this technology has a significant advantage: it can measure the viral load in a sample. This trait might be used to estimate a patient’s contagiousness, for example. On the other hand, molecular assays enhance the viral genetic material in order to identify it. This, by definition, alters the quantity of genetic material present, obviating the possibility of accurately estimating the amount of virus initially present in the sample. Click here to learn some of the best rapid antigen testing tips.
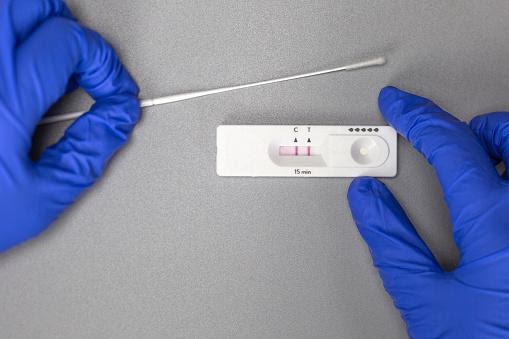
Antigen examinations
Antigen testing identifies another component of the SARS CoV 2 virus, the protein coat that encases the RNA genome. Rapid antigen tests, like molecular testing, are used to identify the presence of viruses in symptomatic or asymptomatic persons and are done on respiratory tract samples. Rapid antigen tests have many benefits over RT-PCR, including their ease of use: they may be conducted at the point of care using a simple swab in contact with the reagent.
Additionally, they are much less expensive, ranging from USD 15 to less than USD 50.6. However, their primary benefit is the speed with which they provide results: most produce findings in 15 to 30 minutes, while RT-PCR takes several hours to run and much longer to get results due to all the pre-and post-analytical work. Thus, quick antigen tests may enable higher testing volume and more rapid separation of individuals who test positive, thus contributing to the breakup of transmission chains sooner.